VITVO at Therapeutic nanoproducts: from biology to innovative technology conference organized by Istituto Superiore di Sanità and AICC on June, 19-20 2019
 Rigenerand is proud to present VITVO at Therapeutic nanoproducts: from biology to innovative technology conference organized by Istituto Superiore di Sanità and AICC on June, 19-20 2019.
Dr. Olivia Candini, R&D Manager of 3D-Culture Technologies division, will have a talk about VITVO applications and potentials on June 20th.
For more infos about the event:
https://www.aicc.website/eventi-aicc/therapeutic-nanoproducts-from-biology-to-innovative-technology/
Rigenerand is proud to present VITVO at Therapeutic nanoproducts: from biology to innovative technology conference organized by Istituto Superiore di Sanità and AICC on June, 19-20 2019.
Dr. Olivia Candini, R&D Manager of 3D-Culture Technologies division, will have a talk about VITVO applications and potentials on June 20th.
For more infos about the event:
https://www.aicc.website/eventi-aicc/therapeutic-nanoproducts-from-biology-to-innovative-technology/Rigenerand VITVO published on Scientific Reports
Medolla – 10 May 2019. Scientific Reports just published the Rigenerand new technology to culture cells in 3D for pre-clinical studies from gene therapy to immunotherapy. “We are proud to present this novel and breakthrough technology on this prestigious academic Journal” says Massimo Dominici MD, Rigenerand founder and scientific coordinator, “VITVO is contributing to fill gaps between in silico hypothesis/in vitro results and in vivo settings also significantly impacting on the 3R rules”. Olivia Candini PhD, the first author of the manuscript and head of Cell and Tissue Engineering R&D, underlines that “VITVO is having a relevant impact in 3D-cultures due to novel features that will empower the most advanced research in cancer additionally boosting other approaches in drug developments”. “Starting from a start-up perspective, Rigenerand is now positioning as leader in the 3D-cultures with published peer-reviewed data that solidly support the proposed technology as tool that allows unprecedented in vitro investigations“ closes Giorgio Mari, Rigenerand CEO. Further Readings Candini O, Grisendi G, Foppiani E, Brogli M, Aramini B, Masciale V, Spano C, Petrachi T, Veronesi E, Conte P, Mari G & Dominici M. A Novel 3D In Vitro Platform for Pre-Clinical Investigations in Drug Testing, Gene Therapy, and Immuno-oncology. Sci Rep. 2019; 9:7154. Available if you like to read itRigenerand scientific founder Massimo Dominici recognized as one of the Global Cell and Gene Therapy Opinion Leaders
 Medolla (Modena) - 03 May 2019. The Medicine Maker a global publication in cell and gene therapy, lists the world’s top 100 thought leaders and opinion shapers who have carried out life-changing work in the biopharmaceutical industry. This year, The Medicine Maker’s Power List 2019 has included Massimo Dominici MD, Rigenerand Scientific Founder and Professor of Oncology at the University of Modena & Reggio Emilia, in the category Champions of Change. “It is really a honor to be recognized in the list amongst such company of global KOL and Nobel Prize winners,” says Dr. Dominici also Past President of the International Society of Cell and Gene Therapy (ISCT) and current Chair of the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapy. The annual Medicine Maker Power List recognizes the most inspiring and influential people across the entire global life sciences sector – from entrepreneurs and CEOs, to industry experts, researchers, and regulators. Reaching a diverse, broad audience around the world, The Medicine Maker is a tier-one publication that celebrates the people, processes and vision that bring new drugs and biologics to the public. The extensive Power List was compiled based on the votes of Medicine Maker readers as well as the input of an expert judging panel made up of influencers in the biopharmaceutical industry.
Related Links
- https://themedicinemaker.com/power-list/2019#champions%20of%20change
- https://www.celltherapysociety.org/news/448122/3-ISCT-Leaders-Recognized-as-Global-Cell-and-Gene-Therapy-Influencers.htm
- https://finance.yahoo.com/news/3-isct-leaders-recognized-global-090000112.html
- https://eprnews.com/3-isct-leaders-recognized-as-global-cell-and-gene-therapy-influencers-403804
Medolla (Modena) - 03 May 2019. The Medicine Maker a global publication in cell and gene therapy, lists the world’s top 100 thought leaders and opinion shapers who have carried out life-changing work in the biopharmaceutical industry. This year, The Medicine Maker’s Power List 2019 has included Massimo Dominici MD, Rigenerand Scientific Founder and Professor of Oncology at the University of Modena & Reggio Emilia, in the category Champions of Change. “It is really a honor to be recognized in the list amongst such company of global KOL and Nobel Prize winners,” says Dr. Dominici also Past President of the International Society of Cell and Gene Therapy (ISCT) and current Chair of the ISCT Presidential Task Force on the Use of Unproven and/or Unethical Cell and Gene Therapy. The annual Medicine Maker Power List recognizes the most inspiring and influential people across the entire global life sciences sector – from entrepreneurs and CEOs, to industry experts, researchers, and regulators. Reaching a diverse, broad audience around the world, The Medicine Maker is a tier-one publication that celebrates the people, processes and vision that bring new drugs and biologics to the public. The extensive Power List was compiled based on the votes of Medicine Maker readers as well as the input of an expert judging panel made up of influencers in the biopharmaceutical industry.
Related Links
- https://themedicinemaker.com/power-list/2019#champions%20of%20change
- https://www.celltherapysociety.org/news/448122/3-ISCT-Leaders-Recognized-as-Global-Cell-and-Gene-Therapy-Influencers.htm
- https://finance.yahoo.com/news/3-isct-leaders-recognized-global-090000112.html
- https://eprnews.com/3-isct-leaders-recognized-as-global-cell-and-gene-therapy-influencers-403804Rigenerand: "Lottiamo contro il cancro" - Il Resto del Carlino 26/02/2019
Premio Mascagni, opera nel distretto biomedicale Modenese NELLA ‘Silicon Valley’ della Bassa modenese, secondo polo al mondo del settore biomedicale, che nemmeno il sisma di maggio 2012 è riuscito a scalfire sebbene passi alla storia come ‘primo terremoto economico d’Italia’, si crea il futuro dei farmaci anti-cancro. Rigenerand, classico esempio di azienda innovativa, è nata dal connubio tra gli oncologi dell’Università di Modena e l’imprenditoria biomedicale, che da sola contribuisce al 2% del Pil nazionale. Fondata nel 2009 come spin off dal manager Gianni Bellini, a capo di RanD e inventore del Performer, apparecchiatura polifunzionale, unica al mondo, per la cura dei tumori e supporto epatico, e dagli oncologi Massimo Dominici e Pierfranco Conte di Unimore, Rigenerand, che opera nel settore dei medical device e delle terapie avanzate, decolla come impresa nel 2016 e in soli tre anni sta facendo grandi passi avanti. L’ad Giorgio Mari spiega la ‘mission’ di Rigenerand: ricerca, innovazione, produzione di farmaci e dispositivi in un settore in grande crescita come quello della diagnostica e delle terapie cellulari. Dottor Mari, Rigenerand è la speranza nella cura dei tumori ancora incurabili? «Se così fosse avremmo davvero raggiunto ciò che il mondo scientifico internazionale auspica, ovvero sconfiggere ogni tipologia di tumori. Meglio, quindi, parlare di importante contributo alla ricerca e in particolare allo sviluppo di nuovi farmaci anticancro e agli innovativi approcci diagnostici capaci di prevedere la risposta alle nuove cure come gli inibitori del check point usati nella immunoterapia». Più che biomedicale vi definite azienda farmaceutica, è così? «L’uno e l’altro, ma sicuramente siamo la prima azienda farmaceutica nata all’interno del distretto biomedicale. Stiamo mettendo a punto una innovativa cura con cellule staminali del paziente stesso, ‘armate’ per produrre una potente molecola anticancro che, somministrata con chemioterapia, possa ridurre la massa tumorale consentendone l’operabilità». Quali riscontri hanno dato i dati pre-clinici? «Sicuramente importanti come il parere positivo circa la definizione di ‘farmaco-orfano’ per la cura del tumore pancreatico localmente avanzato sia da parte dell’Ema (Agenzia europea del farmaco) sia dell’americana Fda (Food and drug administration). Un risultato che ci consente di guardare avanti con ottimismo circa la possibilità di trattare pazienti ancora gravati da una prognosi infausta». Una strategia di cura vincente. «Sì, ma non è l’unica. Si associa infatti all’altra anima della società: sviluppare, produrre, commercializzare sistemi di coltura tridimensionali, detti appunto 3D, che, caricati con cellule, possono predire la risposta ai farmaci riducendo il ricorso alla sperimentazione animale. Un nostro prodotto, Vitvo, è già sul mercato e conta su distributori nazionali e internazionali e sta riscuotendo grande interesse non solo in ambito universitario ma anche tra le aziende farmaceutiche del settore». Link all'articoloRigenerand Gene Therapy Key Technology Published in Scientific Journals
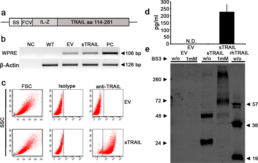
PRESS RELEASE 1 - 2019 Medolla – 19 February 2019 Scientific Reports just publishes the anti-pancreatic cancer gene therapy approach based on modified adipose-derived cells capable to release a novel TRAIL variant with a potent anti-tumor approach. The study, performed in collaboration with the Division of Oncology, Plastic Surgery and Pathology of the University Hospital of Modena and Reggio Emilia and with the laboratory of the Mirandola Technopole, shows for the first time that a novel engineered TRAIL released by human adipose cells can target a variety of human pancreatic cancer cells demonstrating pre-clinical safety and efficacy. The Scientific Report publication parallels an additional piece of scientific information just published on Theranostics regarding the possibility to combine chemotherapy agents and gene delivery for synergies to counteract possible mechanisms of resistance also impacting on tumor stromal cells as key players pancreatic cancer development. This work paves the way towards the clinical translation of the technology in humans within a Phase I/II trial expected for the coming year. “We are extremely excited about these scientific achievements that also arrive after encouraging regulatory feedbacks collected in the last part of 2018”- says Massimo Dominici MD, Rigenerand co-founder and scientific coordinator - “It surely represents a very good start for 2019 indicating the quality of the RR001 platform within our upcoming therapeutic strategy”. Further Readings Spano C, Grisendi G, Golinelli G, Rossignoli F, Prapa M, Bestagno M, Candini O, Petrachi T, Recchia A, Miselli F, Rovesti G, Orsi G, Maiorana A, Manni P, Veronesi E, Piccinno MS, Murgia A, Pinelli M, Horwitz EM, Cascinu S, Conte P, Dominici M. Soluble TRAIL Armed Human MSC As Gene Therapy For Pancreatic Cancer. Sci Rep. 2019;9:1788. Available if you like to read it Rossignoli F, Spano C, Grisendi G, Foppiani EM, Golinelli G, Mastrolia I, Bestagno M, Candini O, Petrachi T, Recchia A, Miselli F, Rovesti G, Orsi G, Veronesi E, Medici G, Petocchi B, Pinelli M, Horwitz EM, Conte P, Dominici M. MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma. Theranostics 2019; 9:436. Available if you like to read it Safe Harbor. This release may contain forward-looking statements regarding RR001 and its future events and results that involve inherent risks and uncertainties. The words “expect,” “intend,” “plan” and similar expressions, as they relate to RR001 or its management, are intended to identify forward-looking statements. Important factors, many of which are beyond the control of Rigenerand srl, could cause actual results to differ materially from those set forth in the forward-looking statements. Rigenerand srl does not assume any obligation to update any of these forward-looking statements.Rigenerand Announces Orphan Drug Designations from both EMA and FDA Obtained by RR001 for the Treatment of Pancreatic Cancer
Rigenerand srl announces the positive opinion on orphan drug designation (ODD) issued by the European Medicines Agency (EMA) for RR001 as treatment of pancreatic cancer. This relevant milestone immediately follows the ODD granted by the FDA (USA) on May 2018 for the same indication. RR001 is a gene therapy product based on adipose derived cells stably modified to produce pro-apoptotic ligands. RR001 is expected to reach the clinical phase within a planned Phase I/II trial as a first-line treatment in combination with chemotherapy in subsets of patients with pancreatic cancer. In addition to provide an access to incentives (e.g. in the EU: fee reductions, no limitation of protocol assistance, possibility to request a conditional approval and specific research grants), the ODD is the recognition by the Agencies of the plausibility of Rigenerand therapeutic approach as well as of its potential to bring in significant benefit to the existing treatments. Rigenerand srl, originally a spin-off by a joint venture between the University of Modena and Reggio Emilia and Rand srl (Italy), fights against cancer by gene modified effector cells and by developing novel 3D culture diagnostic tools. ODD released by key regulatory Agencies represents a boost for the development of RR001 against a still lethal tumor. Rigenerand is very pleased to release the news during the 2018 World Pancreatic Cancer day (http://www.worldpancreaticcancerday.org) that raises the awareness on the unacceptable prognosis of pancreatic cancer calling for global action in better diagnostics and therapeutics. Safe Harbor. This release may contain forward-looking statements regarding RR001 and its future events and results that involve inherent risks and uncertainties. The words “expect,” “intend,” “plan” and similar expressions, as they relate to RR001 or its management, are intended to identify forward-looking statements. Important factors, many of which are beyond the control of Rigenerand srl, could cause actual results to differ materially from those set forth in the forward-looking statements. Rigenerand srl does not assume any obligation to update any of these forward-looking statements. For more info: info@rigenerand.itRigenerand is presenting VITVO at the AACR meeting in Chicago on April, 14/18 2018
Visit us at booth 4207 and explore the VITVO® infinite possibilities of culturing in the 3D dimension.