Covid: cura con le cellule staminali per spegnere il virus Sars-CoV-2. Via ai test
Lo studio partirà a breve e sarà coordinato dall'Ausl di Modena e UniMoRe. L'infusione dovrebbe bloccare l'infiammazione polmonare, ridurre la permanenza in terapia intensiva e i danni a lungo termine
Modena, 26 gennaio 2021 - Per curare il Covid sono stati utilizzati diversi farmaci e varie cure sono state sperimentate in questi mesi di pandemia, ma nessuna di queste ha finora dimostrato di essere determinante. Da oggi c’è un motivo in più per credere che questo maledetto virus potrà essere sconfitto. Dai laboratori del Policlinico di Modena, infatti, è iniziata una sperimentazione che prevede l’utilizzo di cellule staminali (video): "Un ulteriore tentativo, forse più potente di quelli finora tentati, per cercare di spegnere quell’infiammazione che è la causa di una sequenza di eventi che porta al crash del sistema respiratorio, al rischio delle complicazioni e purtroppo alla morte del paziente". Lo spiega il direttore della Struttura complessa di malattie dell’apparato respiratorio dell’Azienda ospedaliero-universitaria di Modena, Enrico Clini (video), che fa parte della cordata di aziende e ospedali chiamati ad applicare su una sessantina di pazienti quello che è emerso dagli studi. Tempo sei mesi per capire se questo tipo di cura avrà ottenuto i suoi risultati. «Per la prima volta in Italia – dice Massimo Dominici (video), direttore della Struttura complessa di oncologia dell’Azienda ospedaliero-universitaria di Modena – abbiamo avuto la forza di mettere assieme cinque strutture che producono cellule per sottoscrivere un unico protocollo clinico, con delle caratteristiche ben definite per quanto riguarda il paziente e con una unicità legata al fatto che potremo confrontare i tipi diversi di cellule all’interno della sperimentazione clinica controllata». Oltre al Policlinico di Modena – che è il coordinatore del progetto Rescat – sono coinvolti gli ospedali Meyer e Careggi di Firenze, il Policlinico Irccs Ca’ Granda di Milano con l’Ospedale Covid di Milano Fiera, l’ospedale San Gerardo di Monza con la Fondazione Centro di ricerca Tettamanti e con l’università Milano-Bicocca, l’azienda ospedaliera universitaria integrata di Verona e l’azienda ospedaliera di Vicenza.
Continua a leggere>
«Per la prima volta in Italia – dice Massimo Dominici (video), direttore della Struttura complessa di oncologia dell’Azienda ospedaliero-universitaria di Modena – abbiamo avuto la forza di mettere assieme cinque strutture che producono cellule per sottoscrivere un unico protocollo clinico, con delle caratteristiche ben definite per quanto riguarda il paziente e con una unicità legata al fatto che potremo confrontare i tipi diversi di cellule all’interno della sperimentazione clinica controllata». Oltre al Policlinico di Modena – che è il coordinatore del progetto Rescat – sono coinvolti gli ospedali Meyer e Careggi di Firenze, il Policlinico Irccs Ca’ Granda di Milano con l’Ospedale Covid di Milano Fiera, l’ospedale San Gerardo di Monza con la Fondazione Centro di ricerca Tettamanti e con l’università Milano-Bicocca, l’azienda ospedaliera universitaria integrata di Verona e l’azienda ospedaliera di Vicenza.
Continua a leggere>Bone Therapeutics and Rigenerand sign partnership for cell therapy process development
Gosselies, Belgium and Modena, Italy, 14 January 2021, 7am CET – BONE THERAPEUTICS (Euronext Brussels and Paris: BOTHE), the cell therapy company addressing unmet medical needs in orthopedics and other diseases, and Rigenerand SRL, the biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology, today announce the signing of a first agreement for a process development partnership. “Allogeneic mesenchymal stem cell (MSC) therapies are currently being developed and evaluated in numerous clinical studies covering diverse therapeutic areas such as musculoskeletal, lung, liver, cardiovascular and autoimmune diseases at an incredible pace. Advances in process development to scale up these therapies could have major impacts for both their approval and commercial viability. This will be essential to bring these therapies to market to benefit patients as quickly as possible,” said Miguel Forte, CEO, Bone Therapeutics. “Hence, whilst Bone Therapeutics is driving on its existing clinical development programs, we have signed a first formal agreement with Rigenerand as a fellow MSC-based organization. This will result in both companies sharing extensive expertise in the process development and manufacturing of MSCs and cell and gene therapy medicinal products. Bone Therapeutics also selected Rigenerand to partner with for their additional experience with wider process development of advanced therapy medicinal products (ATMPs), including the conditioning and editing of MSCs. Rigenerand was founded by Massimo Dominici, a world opinion leader in the cell therapy with an unparalleled MSC expertise and knowledge.” The scope of collaborations between Bone Therapeutics and Rigenerand aims to focus on different aspects of product and process development for Bone Therapeutics’ expanding therapeutic portfolio. Rigenerand will contribute to improving the processes involved in the development and manufacture of Bone Therapeutics’ MSC based allogeneic differentiated cell therapy products as they advance towards patients. The first collaboration between the two organizations will initially focus on augmented professional bone-forming cells – cells that are differentiated and programmed for a specific task. There is also potential for Bone Therapeutics to broaden its therapeutic targets and explore new mechanisms of action with potential gene modifications for its therapeutic portfolio. In addition to Rigenerand’s MSC expertise, Bone Therapeutics also selected Rigenerand as a partner for Rigenerand’s GMP manufacturing facility. This facility, situated in Modena, Italy, has been designed to host a number of types of development processes for ATMPs. These include somatic, tissue engineered and gene therapy processes. These multiple areas of Rigenerand capabilities enable critical development of new processes and implementation of the gene modification of existing processes. In addition, Rigenerand has built considerable experience in cGMP manufacturing of MSC-based medicinal products, including those that are genetically modified. “Process development and manufacturing is a key part of the development for ATMPs internationally. Navigating these therapies through the clinical development phase and into the market requires a carefully considered process development pathway,” said Massimo Dominici, scientific founder, Rigenerand, professor of medical oncology, and former President of the International Society for Cell & Gene Therapy (ISCT). “This pathway needs to be flexible, as both the market and materials of these therapies continues to evolve alongside an improved clinical efficacy.” “Rigenerand will offer considerable input from its experience of MSC-based therapies to enable Bone Therapeutics to keep and further accelerate the pace in development of the product processes of its MSC based allogeneic differentiated cell therapy as they advance towards patients,” said Giorgio Mari, CEO, Rigenerand. “We will continue to use our MSC expertise in the development of Rigenerand’s own products, as well as in process development and manufacturing cell and gene therapies for partner organizations across the globe.” About Bone Therapeutics Bone Therapeutics is a leading biotech company focused on the development of innovative products to address high unmet needs in orthopedics and other diseases. The Company has a, diversified portfolio of cell and biologic therapies at different stages ranging from pre-clinical programs in immunomodulation to mid-to-late stage clinical development for orthopedic conditions, targeting markets with large unmet medical needs and limited innovation. Bone Therapeutics is developing an off-the-shelf next-generation improved viscosupplement, JTA-004, which is currently in phase III development for the treatment of pain in knee osteoarthritis. Consisting of a unique combination of plasma proteins, hyaluronic acid - a natural component of knee synovial fluid, and a fast-acting analgesic, JTA-004 intends to provide added lubrication and protection to the cartilage of the arthritic joint and to alleviate osteoarthritic pain and inflammation. Positive phase IIb efficacy results in patients with knee osteoarthritis showed a statistically significant improvement in pain relief compared to a leading viscosupplement. Bone Therapeutics’ core technology is based on its cutting-edge allogeneic cell therapy platform with differentiated bone marrow sourced Mesenchymal Stromal Cells (MSCs) which can be stored at the point of use in the hospital. Currently in pre-clinical development, BT-20, the most recent product candidate from this technology, targets inflammatory conditions, while the leading investigational medicinal product, ALLOB, represents a unique, proprietary approach to bone regeneration, which turns undifferentiated stromal cells from healthy donors into bone-forming cells. These cells are produced via the Bone Therapeutics’ scalable manufacturing process. Following the CTA approval by regulatory authorities in Europe, the Company is ready to start the phase IIb clinical trial with ALLOB in patients with difficult tibial fractures, using its optimized production process. ALLOB continues to be evaluated for other orthopedic indications including spinal fusion, osteotomy, maxillofacial and dental. Bone Therapeutics’ cell therapy products are manufactured to the highest GMP (Good Manufacturing Practices) standards and are protected by a broad IP (Intellectual Property) portfolio covering ten patent families as well as knowhow. The Company is based in the BioPark in Gosselies, Belgium. Further information is available at www.bonetherapeutics.com. About Rigenerand Rigenerand SRL is a biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology and 3D bioreactors as alternative to animal testing for pre-clinical investigations. Rigenerand operates through three divisions: 1) a proprietary pipeline, developing and GMP manufacturing of cell and gene therapies for cancer treatment, 2) a CDMO division, providing GMP support for scale-up of cell based medicinal products for clinical and commercial purposes within fully equipped Grade A/B cleanrooms, and 3) a division developing 3D technologies for cell culture, developing and manufacturing 3D solutions for R&D diagnostics and pre-clinical purposes (VITVO®). Rigenerand is developing RR001, a proprietary ATMP gene therapy medicinal product for the treatment of pancreatic ductal adenocarcinoma (PDAC). RR001 has been granted an Orphan Drug Designation (ODD) by US-FDA and from the European Medicine Agency. The Clinical trial is expected to start in Q2 2021. Rigenerand is headquartered in Medolla, Modena, Italy, with more than 1,200 square metres of offices, R&D and quality control laboratories and a cell factory of 450 square metres of sterile cleanroom (EuGMP Grade-B) with BSL2/BSL3 suites for cell and gene therapies manufacturing. It combines leaders and academics from biopharma and medical device manufacturing sectors. Contact Information: Image Box PR Neil Hunter / Michelle Boxall Tel +44 (0)20 8943 4685 neil@ibcomms.agency / michelle@ibcomms.agencyGlobal Cell Culture Market Trends: Product Development For 3D Cell Culture
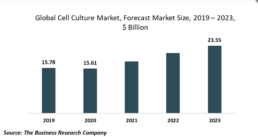
The Business Research Company’s Global Cell Culture Market Report 2020-30: Covid 19 Growth And Change LONDON, GREATER LONDON, UK, October 12, 2020 /EINPresswire.com/ -- The development of products for 3D cell culture by companies operating in the cell culture market is gaining significant popularity. A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions, similar to how they would in a living organism. For instance, in July 2020, Rigenerand, an Italian company engaged in the production of innovative cell-based technology products for regenerative medicine and oncology, launched a 3D bioreactor called VITVO handheld bioreactor to offer increased usability, sizing, closed system, and flexibility for 3D cell culture technologies. Similarly, Jellagen, a UK-based marine biotechnology company, launched JellaGel, a jellyfish collagen hydrogel, suitable for 3D cell culture and tissue engineering.Rigenerand commences 3D bioreactor development for cGMP extracellular vesicle exosomes (EV) production
Development supported by part of Horizon 2020 consortium grant Modena, Italy, July 8, 2020 - Rigenerand SRL, the biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology, today announces it has commenced development of a novel 3D bioreactor and pipeline, specifically designed for the incremental production of extracellular vesicles exosomes (EVs) in a cGMP environment. Rigenerand’s project development will be supported with part of a grant of EUR 4.4 million from Horizon 2020 for the H2020-EU.1.2.2.-FET Proactive program, awarded to the Biogenic Organotropic Wetsuits project. This project comprises a consortium of companies involving Rigenerand. The Biogenic Organotropic Wetsuits (Grant Agreement ID: 952183) project involves eleven organizations across seven countries in Europe. Rigenerand will contribute to the consortium its combination of expertise, technology and innovation focused on biomaterials, prototypes production and industrialization of cell culture devices and 3D cell cultures. Rigenerand will start developing novel ways of improving current EV cGMP production technologies. These technologies ultimately aim to EV clinical batches to be used as drug substances for cell-free therapies medicinal products. The project is due to start activity in Q4 2020. By Q2 2021, Rigenerand is planning to have developed the isolation and expansion of a human cellular source (EDT-MSC) suitable for the production of immune-ghost EVs as well as the separation and production of suitable amounts of EV for characterization and pre-clinical application. “This project will contribute to the urgent medical need of increasing EV production. It will enable Rigenerand to develop pre-conditioning approaches that are critical to providing solutions to these challenges,” said Massimo Dominici, scientific founder, Rigenerand. “The activity for this consortium will also enable Rigenerand to develop platforms for new therapeutic solutions, covering a range of clinical applications, including oncology and regenerative medicine to medical devices.” The Rigenerand 3D bioreactor for EV production will be based on its proprietary VITVO technology. VITVO is a handheld bioreactor, with an integrating scaffold for the establishment of a 3D cell culture model. It is already used for highly predictive pre-clinical testing. It has been specifically designed to offer an increase to the market in usability, sizing, closed system, and flexibility for 3D cell culture technologies. VITVO has been extensively used for pre-clinical development of its autologous gene therapy medicinal product RR001, contributing to evaluation of the action and efficacy of the drug. It also allows the reduction of the number of animals employed in the experimentation for dose finding studies. VITVO has also been used in a variety of assays focusing on oncology. The efficacy of chemotherapy, biologics and cell-based anti-cancer agents has been tested by comparing VITVO with an in vivo preclinical xeno-transplant model. Notably, the system was challenged using primary tumor cells harvested from lung cancer patients as an innovative predictive functional assay for cancer responsiveness to a checkpoint inhibitor, such as nivolumab. The results suggest VITVO as a 3D in vitro model for pre-clinical testing with a possible relevant impact in other areas external to oncology. “Despite a high demand in the market for 3D cell culture models to improve increased information compared to 2D models, 3D models are not yet routinely deployed,” said Giorgio Mari, Rigenerand CEO. “We will continue to develop 3D technologies, and maintain our market leading position by increasing collaborations with HTS technology developers and pharmaceutical firms who have technology that can work with VITVO. We are also collaborating with academic research units to develop new applications.” About Rigenerand Rigenerand SRL is a biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology and 3D bioreactors as alternative to animal testing for pre-clinical investigations. Rigenerand operates through three divisions: 1) a proprietary pipeline, developing and GMP manufacturing of cell and gene therapies for cancer treatment, 2) a CDMO division, providing GMP support for scale-up of cell based medicinal products for clinical and commercial purposes within fully equipped Grade A/B cleanrooms, and 3) a division developing 3D technologies for cell culture, developing and manufacturing 3D solutions for R&D diagnostics and pre-clinical purposes (VITVO®). Rigenerand is developing RR001, a proprietary ATMP gene therapy medicinal product for the treatment of pancreatic ductal adenocarcinoma (PDAC). RR001 has been granted an Orphan Drug Designation (ODD) by US-FDA and from the European Medicine Agency. The Clinical trial is expected to start in Q1 2021. Rigenerand is headquartered in Medolla, Modena, Italy, with more than 1,200 square metres of offices, R&D and quality control laboratories and a cell factory of 450 square metres of sterile cleanroom (EuGMP Grade-B) with BSL2/BSL3 suites for cell and gene therapies manufacturing. It combines leaders and academics from biopharma and medical device manufacturing sectors. Contact Information: Image Box PR Neil Hunter / Michelle Boxall Tel +44 (0)20 8943 4685 neil@ibcomms.agency / michelle@ibcomms.agencyMassimo Dominici, scientific founder of Rigenerand, in the Power List 2020 of World Opinion Leaders for Advanced Medicine (ATMP)
 Rigenerand’s founder, Massimo Dominici MD, has been listed once again by The Medicine Maker, leading international magazine for pharmaceutical development, as one of the 60 best leaders and opinion makers in the industry. Prof. Massimo Dominici is the only Italian of the 20 honored in the category Advanced Medicine this year (which includes therapies based on cells, also genetically modified ones). For Prof. Dominici this is an affirmation as he had been already listed in 2019.
The Medicine Maker is a leading international magazine for cell and gene therapy that creates an annual list of the world’s 60 best leaders and opinion makers whose work has contributed to a significant progress in the biopharmaceutical industry and therefore in medicine.
“I am honored by this recognition – commented Prof. Massimo Dominici – that celebrates the work accomplished by me and my coworkers in the Laboratories for Cell Therapy Research of the University Hospital Modena and the company Rigenerand. Our goal is to transfer years of research on cells to various branches of medicine and surgery, also thanks to a wide range of international collaborations. Our vocation is to serve as platform for the development of cell therapies by ourselves and as CDMO for all of those who believe in cells as medicinal products.”
The Power List of The Medicine Maker is created by a group of experts who analyze the work done in the industry, the visibility and the number of citations of the scientific pubblications of those authors. This year’s categories were Small Molecules, Advanced Medicine and Biopharmaceuticals, with 20 leaders for each category.
https://themedicinemaker.com/power-list/2020
Rigenerand’s founder, Massimo Dominici MD, has been listed once again by The Medicine Maker, leading international magazine for pharmaceutical development, as one of the 60 best leaders and opinion makers in the industry. Prof. Massimo Dominici is the only Italian of the 20 honored in the category Advanced Medicine this year (which includes therapies based on cells, also genetically modified ones). For Prof. Dominici this is an affirmation as he had been already listed in 2019.
The Medicine Maker is a leading international magazine for cell and gene therapy that creates an annual list of the world’s 60 best leaders and opinion makers whose work has contributed to a significant progress in the biopharmaceutical industry and therefore in medicine.
“I am honored by this recognition – commented Prof. Massimo Dominici – that celebrates the work accomplished by me and my coworkers in the Laboratories for Cell Therapy Research of the University Hospital Modena and the company Rigenerand. Our goal is to transfer years of research on cells to various branches of medicine and surgery, also thanks to a wide range of international collaborations. Our vocation is to serve as platform for the development of cell therapies by ourselves and as CDMO for all of those who believe in cells as medicinal products.”
The Power List of The Medicine Maker is created by a group of experts who analyze the work done in the industry, the visibility and the number of citations of the scientific pubblications of those authors. This year’s categories were Small Molecules, Advanced Medicine and Biopharmaceuticals, with 20 leaders for each category.
https://themedicinemaker.com/power-list/2020