GEN News: 3D Bioreactor Shows Promise for Donor Cell Therapies
A company has developed an iPhone-like bioreactor that may have the potential to aid quality control to validate the efficacy of cell therapies. The flat bioreactor, which has an inner section to hold various cell types, is designed to give a real-time 3D view of the tumor environment.Read moreThe Power List 2021: Massimo Dominici
Professor at the University Hospital of Modena and Reggio Emilia; and Scientific Founder of Rigenerand
“COVID-19 has led to challenges with clinical trials; almost 1500 trials have reported delays due to the pandemic. This is particularly true for phase I studies.
Continue>Produzione industriale di medicinali a base di cellule in accordo alle cGMP: sfide e soluzioni
by S. Guidi Negli ultimi anni la produzione di medicinali a base di cellule vitali si sta spostando dalla tradizionale Clean Room, simile al laboratorio in cui sono stati storicamente sviluppati, a tecnologie industriali già note nell’industria farmaceutica per la produzione di medicinali biologici. Per garantire la disponibilità di questi medicinali preziosi ad un numero crescente di pazienti, il passaggio ad una tipologia di produzione industriale è necessaria, tenendo conto delle peculiarità di un prodotto estremamente delicato e difficilmente standardizzabile.Quando le cellule sono diventate farmaci Le cellule sono state impiegate con grande successo nella pratica clinica ben prima che venissero classificate come Medicinali per Terapia Avanzata (ATMPs). Una storia esemplare di questo successo è la storia della cute artificiale, che era già stata utilizzata su migliaia di pazienti prima che venisse definita medicinale di ingegneria tessutale. continue reading
Rigenerand is proud to announce that PELOBiotech joined the European Sales Network as distributor of VITVO®
PELOBiotech GmbH is proud to announce that with Rigenerand SRL, Italy, we were able to sign up a very innovative partner in the field of 3D Cell Culture models. PELOBiotech will exclusively distribute their bioreactor VITVO® in Belgium, DACH, Denmark, France, the Netherlands and UK/Ireland. VITVO® completes PELOBiotech’s comprehensive portfolio of 3D Cell Culture models and systems. „I am happy to add this innovative and already successful tested 3D system VITVO to our 3D technologies division. 3D models are gaining more and more interest now in every research area, especially in the personalized medicine sector. It will make a huge difference for the European research“, says Dr. Peter Frost, CEO PELOBiotech GmbH, Germany. PELOBiotech is known for its intensive competence and broad portfolio of 3D models, systems & tools. VITVO® is a ready to use, flat and handheld bioreactor, integrating scaffold for the establishment of in vitro 3D cell culture model that can be used for a large number of research applications and pre-clinical investigations. This in vitro 3D cell culture technology offers also a solution for precision medicine approaches. The innovative 3D in-vitro-Model mimics the in-vivo complexity. Cells grow in a 3D manner enabling a real in vitro reconstruction of the tissue structure. Rigenerand has developed VITVO® as an innovative handheld bioreactor with an integrated scaffold for establishing an in vitro 3D cell culture model. VITVO’s 3D models will greatly enhance academic and pharma research and pre-clinical programs for cell and gene therapies VITVO® is already used for highly predictive pre-clinical testing as it has been specifically created to offer an increase to the market in usability, sizing, closed system, and flexibility for 3D cell culture technologies. VITVO®’s standardized 3D platform includes a contained in closed, transportable device that minimizes contamination. VITVO® will also ensure to offer a technology compliant with 3R principals, assisting to reduce the animal use in pre-clinical testing and for dose finding studies. Rigenerand developed and tested VITVO® for pre-clinical development for the treatment of solid tumors. If you like to know more about Rigenerand SRL Rigenerand is a biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology and 3D bioreactors as alternative to animal testing for pre-clinical investigations. It is very active in the GMP manufacturing of cell and gene therapies for cancer treatment. division developing 3D technologies for cell culture, developing and manufacturing 3D solutions for R&D diagnostics and pre-clinical purposes (VITVO®). Rigenerand is located in Medolla, Modena, Italy, with its offices, R&D and quality control laboratories and a cell factory with sterile cleanroom with BSL2/BSL3 suites for cell and gene therapies manufacturing. It combines leaders and academics from biopharma and medical device manufacturing sectors. See moreNovember 19, 2020 - World pancreatic Cancer Day
World pancreatic Cancer Day falls on the third thursday every year, which is November 19 in 2020. This is a day where people around the world will unite to Demand Better in the fight against the world’s toughest cancer. The World Pancreatic Cancer Coalition has brought together more than 60 organizations from 27 countries and six continents to raise awareness and inspire action on World Pancreatic Cancer Day. Through this combined effort, we are bringing greater attention, awareness, and better outcomes to this deadly disease. >> https://www.awarenessdays.com/awareness-days-calendar/world-pancreatic-cancer-day-2020/
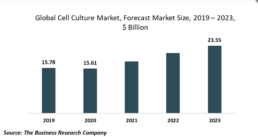
Global Cell Culture Market Trends: Product Development For 3D Cell Culture
The Business Research Company’s Global Cell Culture Market Report 2020-30: Covid 19 Growth And Change LONDON, GREATER LONDON, UK, October 12, 2020 /EINPresswire.com/ -- The development of products for 3D cell culture by companies operating in the cell culture market is gaining significant popularity. A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions, similar to how they would in a living organism. For instance, in July 2020, Rigenerand, an Italian company engaged in the production of innovative cell-based technology products for regenerative medicine and oncology, launched a 3D bioreactor called VITVO handheld bioreactor to offer increased usability, sizing, closed system, and flexibility for 3D cell culture technologies. Similarly, Jellagen, a UK-based marine biotechnology company, launched JellaGel, a jellyfish collagen hydrogel, suitable for 3D cell culture and tissue engineering.genengnews.com: Improving Cell Therapy’s Supply Chain
 Several elements of making cell therapies create significant obstacles in getting these treatments to more patients.
“A main challenge for cell therapies—autologous or allogeneic—is cryopreservation in the supply chain,” says Simona Guidi, business development director at Rigenerand. “Products have a very short shelf life, sometimes hours or days.”
That obstacle prevents many patients from getting such treatments. A potential solution involves getting the production closer to patients. “That is a big issue,” points out Guidi.
Keep reading
Several elements of making cell therapies create significant obstacles in getting these treatments to more patients.
“A main challenge for cell therapies—autologous or allogeneic—is cryopreservation in the supply chain,” says Simona Guidi, business development director at Rigenerand. “Products have a very short shelf life, sometimes hours or days.”
That obstacle prevents many patients from getting such treatments. A potential solution involves getting the production closer to patients. “That is a big issue,” points out Guidi.
Keep reading
Rigenerand receives regulatory approval for gene therapy production
Authorization enables Rigenerand to enter into clinic and offer CDMO services and consultancy to cell and gene therapy sector
Modena, Italy, June 2, 2020 - Rigenerand SRL, the biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology, today announces it has received authorization from the Italian Medicine Authority (AIFA) to produce gene therapy medicinal products for clinical purposes. This authorization enables Rigenerand to manufacturer its own autologous gene therapy medicinal product (RR001) for the treatment of pancreatic cancer. It also authorizes the company to start its phase I first-in-man clinical trial of RR001, now expected to start in Q1, 2021. RR001 has been granted Orphan Drug Designation (ODD) by both the US FDA (Food and Drug Administration) and European Commission COMP (Committee for Orphan Medicinal Products). This ODD offers the opportunity for Rigenerand to request an accelerated assessment procedure and quicker development pathways towards a marketing authorization in the US and EU. Rigenerand will also now offer direct GMP CDMO services to international and Italian partners in clinical development of cell and gene therapy products (ATMPs). The company will utilise its experience from its drug development and diagnostic arms to deliver a science-based approach to its GMP manufacturing services and cell-based medicinal products development. Rigenerand plans to expand its manufacturing facility from five sterile clean rooms suits. This will be by implementing further closed system and isolator technology in pre-designated areas in its facilities. The five sterile cleanrooms are within the Rigenerand GMP facility, which contains a Biosafety Level 3 (BSL3) negative pressure area, suitable to handle genetically modified microorganisms (MOGM), viruses and Risk Group 3 microorganisms, as well as a Biosafety Level 2 (BSL2), positive pressure area: suitable to manipulate non-infectious cell based products and Risk Group 2 microorganisms. Rigenerand’s cleanroom technology offers the flexibility to scale-up the processes from academic / hospital laboratories, and the feasibility of technology transfer of manufacturing processes from other cell factories in order to expand their process capability. In addition, Rigenerand is now authorized to deliver consultancy to biotechnology and pharmaceutical companies on cell and gene therapy development and manufacturing. This consultancy includes expanding process capabilities and developing early-stage cell and gene therapy medicinal products for clinical purposes. As the cell and gene therapy sector continues to grow, with increased numbers of therapies moving through clinical development and onto commercialization, demand for CDMO services will continue to grow. There is an increased demand for global networks of CDMO GMP cell and gene therapy manufacturing. This calls for an improved capacity to treat patients whilst reducing logistical complexities, issues, risks, and costs. “The regulatory approval for Rigenerand to produce gene therapies for clinical development now enables Rigenerand to enter the clinic with its own gene therapy product to target pancreatic cancer,” said Massimo Dominici, scientific founder, Rigenerand. “Combined with the Orphan Drug Designation, the approval will enable Rigenerand to choose an accelerated pathway to bring a gene therapy approach to pancreatic cancer patients with little alternative therapeutic option.” “The authorization is also essential in allowing Rigenerand to offer its much needed GMP CDMO services to the wider cell and gene therapy sector,” said Giorgio Mari, Rigenerand CEO. “We will be expanding our CDMO facility to cater for increasing demand. Operating as both a developer with a clinical pipeline as well as a CDMO has resulted in an unrivalled blend of expertise for us to offer to partners and the wider cell and gene therapy industry.” About Rigenerand Rigenerand SRL is a biotech company that both develops and manufactures medicinal products for cell therapy applications, primarily for regenerative medicine and oncology and 3D bioreactors as alternative to animal testing for pre-clinical investigations. Rigenerand operates through three divisions: 1) a proprietary pipeline, developing and GMP manufacturing of cell and gene therapies for cancer treatment, 2) a CDMO division, providing GMP support for scale-up of cell based medicinal products for clinical and commercial purposes within fullyequipped Grade A/B cleanrooms, and 3) a division developing 3D technologies for cell culture, developing and manufacturing 3D solutions for R&D diagnostics and pre-clinical purposes (VITVO®). Rigenerand is developing RR001, a proprietary ATMP gene therapy medicinal product for the treatment of pancreatic ductal adenocarcinoma (PDAC). RR001 has been granted an Orphan Drug Designation (ODD) by US-FDA and from the European Medicine Agency. The Clinical trial is expected to start in Q1 2021. Rigenerand is headquartered in Medolla, Modena, Italy, with more than 1,200 square metres of offices, R&D and quality control laboratories and a cell factory of 450 square metres of sterile cleanroom (EuGMP Grade-B) with BSL2/BSL3 suites for cell and gene therapies manufacturing. It combines leaders and academics from biopharma and medical device manufacturing sectors.ESMH - Alternative approaches to animal testing
In 1959, The Principles of Humane Experimental Technique was published by Russell and Burch, introducing the 3Rs concept (replacement, reduction, and refinement) regarding animal use in the scientific community. Just over 60 years later, it is timely to review progress to date and the future outlook regarding alternative approaches to animal testing. The US Environmental Protection Agency (EPA) announced elimination of all mammal study requests and funding by 2035, and the Dutch Minister for Agriculture aims for the Netherlands to become the ‘world leader in innovations without laboratory animals by 2025’. These ambitious objectives by the Netherlands and the US are backed also at an EU level, with both citizens and industry anticipating significant reductions in the coming years.Read more
The US Environmental Protection Agency (EPA) announced elimination of all mammal study requests and funding by 2035, and the Dutch Minister for Agriculture aims for the Netherlands to become the ‘world leader in innovations without laboratory animals by 2025’. These ambitious objectives by the Netherlands and the US are backed also at an EU level, with both citizens and industry anticipating significant reductions in the coming years.Read moreNovember is Pancreatic Cancer Awareness Month
Save the date – World Pancreatic Cancer Day – Nov. 21, 2019!

November is Pancreatic Cancer Awareness Month, when we as a community shine the brightest! It is yet another occasion for us to celebrate our survivors and honor loved ones who have fought this disease. There is also a unique opportunity this month to raise awareness, educate the world by sharing our stories, raise money for research and let patients know that we will never give up.The Hirshberg Foundation strives to guide and embrace each patient, support every family and fund the foremost cutting-edge researchers who will yield results and turn the tide. We accomplish this by funding Seed Grants, providing patient services and giving communities opportunities to unite through our events. We couldn’t do any of it without you, especially in November.
Show your support this month by joining our November awareness campaign: Celebrate, Participate and Dedicate! Each word represents an action you can take to make a difference in the fight against this disease. It remains the Hirshberg Foundation’s heartfelt promise to never give up in this fight against pancreatic cancer, and together, we can fulfill that promise. Below, in our What You Can Do section, learn how to get started!
Pancreatic Cancer Facts
Pancreatic cancer is the 3rd leading cause of cancer-related death in the United States surpassing breast cancer. It is expected to become the 2nd by 2020, surpassing colon cancer.
Every day, more than 1,257 people worldwide will be diagnosed with pancreatic cancer. In nearly every country, pancreatic cancer is the only major cancer with a single-digit five-year survival rate of 2- 9%.
It is estimated that in 2025, 557,688 new cases will be diagnosed globally.
As a member of the World Pancreatic Cancer Coalition we recognize we are not in this fight alone. Our message to Never Give Up goes hand-in-hand with the coalition’s message to Demand Better. We’ve made great strides but pancreatic cancer families affected by this disease deserve a fighting chance. Better progress starts with early detection. The key to early detection is knowing the symptoms and risks for pancreatic cancer. Know the Signs and Symptoms and learn more about patient tools, resources and our educational symposium today.
What You Can Do