The development of new therapeutic approaches based on manipulated cells represents the new frontier for the treatment of tumors, on the side of conventional pharmacological therapies.
Anti-Cancer Gene Therapies
RR001 is a cell-based product for the treatment of solid tumours, for which therapies such as chemotherapy and radiotherapy did not have a significant effect on patients’ health. The product has been classified by European Medicines Agency (EMA) as gene therapy.
RR001 technology is a medicine product based on autologous human adipose perivascular stromal cells genetically modified to secrete soluble tumor necrosis factor-related apoptosis-inducing ligand (AD-PC sTRAIL).
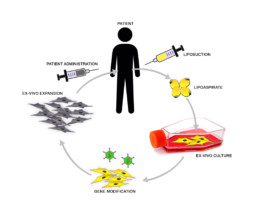
AD-PC isolated from the patient’s adipose tissue, through a minimally invasive liposuction procedure, are in vitro cultured and engineered to stably produce the anti-tumor molecule TRAIL. Once expanded the AD-PC sTRAIL are reinfused into the patient to generate the therapeutic effect.
- Cells are isolated from the patient’s adipose tissue
- Cells are then cultured and expanded ex-vivo, and genetically engineered
- The therapeutic gene inserted within the stromal progenitors induces the constant release of an anti-tumor protein, which is able to kill tumor cells.
- Cells containing the therapeutic gene are then administered to the patient as an antitumoral treatment.
The method for the development of the treatment agent is proprietary
Publications
Spano et al., Soluble TRAIL Armed Human MSC As Gene Therapy For Pancreatic Cancer. Sci Rep. 2019 Feb 11;9(1):1788. doi: 10.1038/s41598-018-37433-6.